QUVIVIQ™ works differently to other hypnotics
Upon discontinuation of QUVIVIQ™, with up to 12 months of continuous treatment, clinical trial data revealed:2

no evidence of abuse or withdrawal symptoms, indicating no physical dependence

no evidence of
rebound insomnia
QUVIVIQ™ once‑nightly dosing
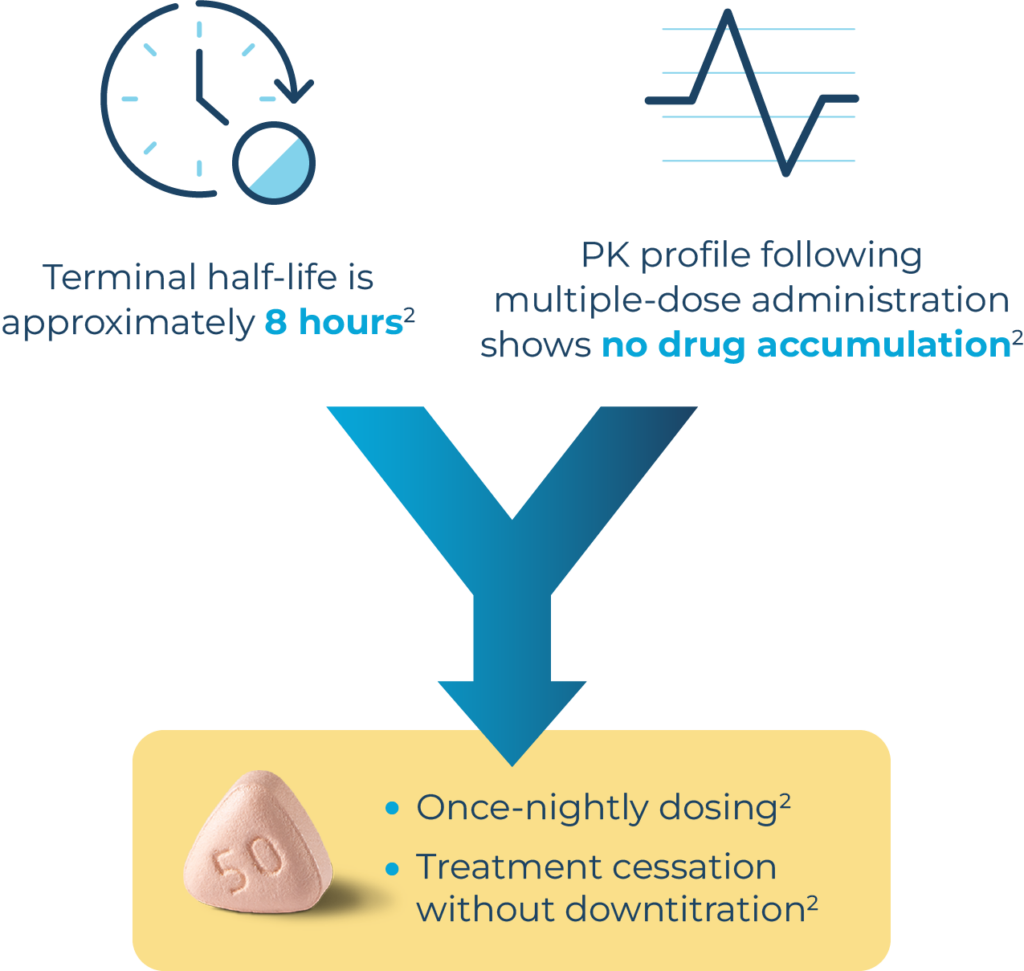
QUVIVIQ™ should be used for as short a time as possible. The appropriateness of continued treatment should be assessed within 3 months and periodically thereafter.2

For patients with moderate hepatic impairment or taking moderate CYP3A4 inhibitors (eg erythromycin, ciprofloxacin or ciclosporin) the recommended QUVIVIQ™ dose is 25 mg once per night.2
Taking QUVIVIQ™
QUVIVIQ™ should be taken each night within 30 minutes before going to bed2
Time to sleep onset may be delayed if taken soon after a large meal2
Patients should continue to practice good sleep hygiene5,6
QUVIVIQ™ is NICE recommended
- CBTi has been tried but not worked, or
- CBTi is not available or is unsuitable
NICE also recommends advising patients on good sleep hygiene. This includes:12
- maintaining a comfortable sleep environment
- keeping regular sleep schedules (going to bed and getting up at similar times, avoiding daytime napping)
- relaxing before going to bed (eg having a bath, reading a book)
- limiting/ avoidance of caffeine, nicotine and alcohol
Over‑the‑counter treatments
Quiz Summary
0 of 1 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Time has elapsed
Categories
- Not categorized 0%
-
Thank you for your response.
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 1
1. Question
Ready for the next step?
5-HT, serotonin; ACh, acetylcholine; ARAS, ascending reticular activating system; CBTi, cognitive behavioural therapy for insomnia; DA, dopamine; DORA, dual orexin receptor antagonist; GABA, gamma-aminobutyric acid; GABA-A, gamma-aminobutyric acid type-A; HA, histamine; MoA, mode of action; NA, noradrenaline; NICE, National Institute for Health and Care Excellence; ORX, orexin; OTC, over the counter; PK, pharmacokinetic
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.2
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- Roch C, Bergamini G et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology 2021;238(10):2693-2708
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- Janto K Prichard J R, Pusalavidyasagar S. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med 2018;14(8):1399-1408
- Saper C B, Scammell T et al. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437(7063):1257-1263
- Chaput J P, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep 2018;10:421-430
- Di Marco T, Djonlagic I et al. Effect of daridorexant on sleep architecture in patients with chronic insomnia disorder: a pooled post hoc analysis of two randomized Phase 3 clinical studies. Sleep 2024:doi.org/10.1093/sleep/zsae1098
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1:doi:10.3389/frsle.2022.935228
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‑blind, placebo‑controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 922. Daridorexant for treating long‑term insomnia, 18 October 2023. Available at: nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing long‑term insomnia (more than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing short-term insomnia (less than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- Culpepper L, Wingertzahn M A. Over-the-counter agents for the treatment of occasional disturbed sleep or transient insomnia: a systematic review of efficacy and safety. Prim Care Companion CNS Disord 2015;17(6):10.4088/PCC.15r01798
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00654 | Date of preparation: September 2025

