Management of the symptoms of chronic insomnia with QUVIVIQ™
There are considerable unmet needs in the clinical management of chronic insomnia,1 such as the need for innovative behavioural and pharmacological treatments that are effective for long-term use2
Beyond first‑line cognitive behavioural therapy for insomnia (CBTi), some people suffering from insomnia require pharmaceutical intervention.3 Some treatments licensed for short‑term insomnia have been associated with reduced sleep quality and daytime somnolence, as well as tolerance or addiction which may be exacerbated by unlicensed longer-term use.3,4
There is a need for treatments which provide:3,4
sufficient efficacy for improving sleep quality
acceptable side‑effect profile
low risk of tolerability/ dependence
an improvement in daytime functioning
The QUVIVIQ™ clinical trial programme was designed to assess both night‑time and daytime impact of chronic insomnia3
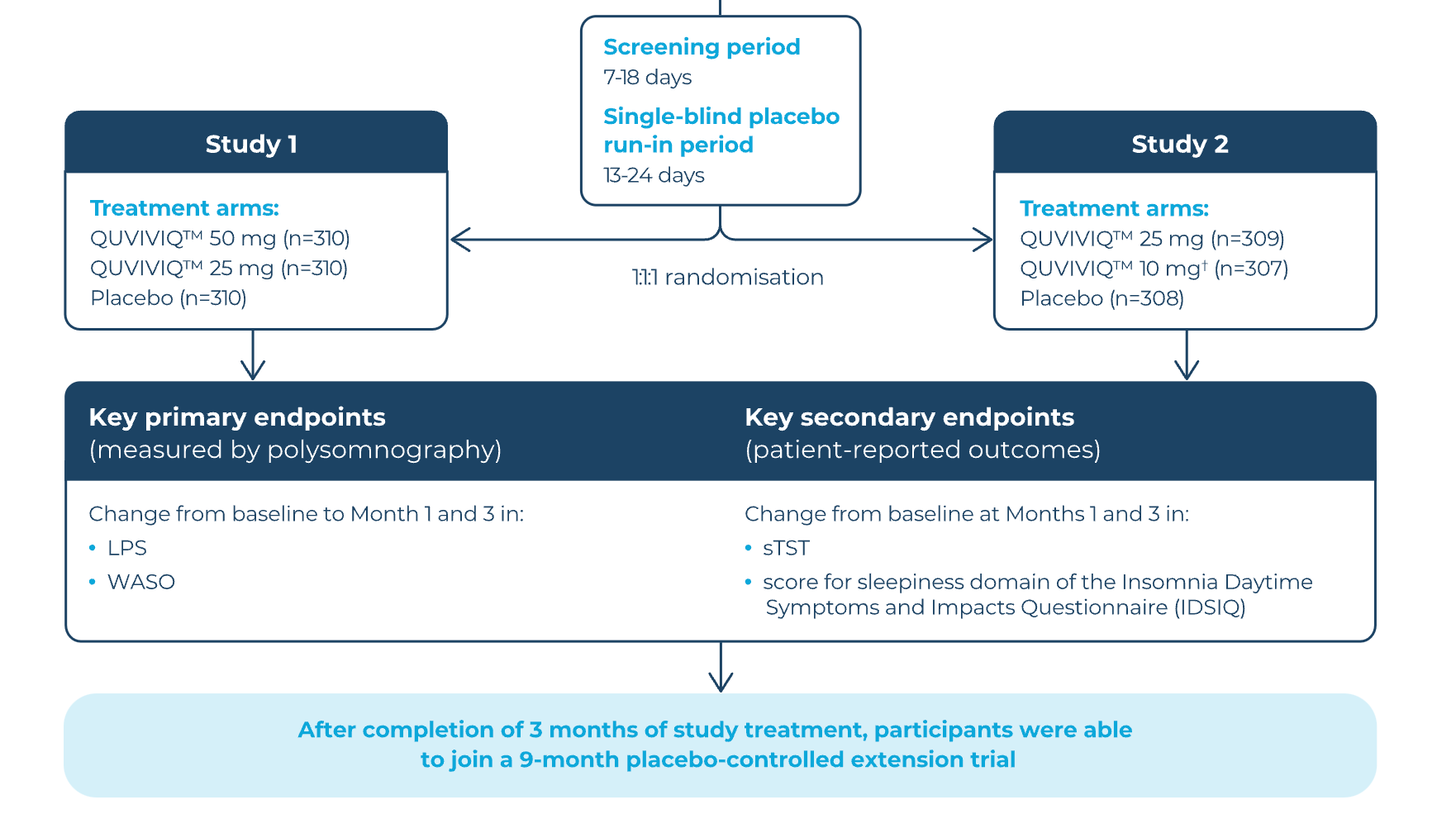
The primary endpoints for these studies were latency to persistent sleep (LPS) and wake time after sleep onset (WASO), as measured by polysomnography.3 While these provide clinically meaningful parameters for the measure of sleep quality, in practice, improvements in sleep will often be based on the subjective experience of the individual patient.5 Secondary endpoints of the studies included self‑reported total sleep time (sTST) and the presence of daytime symptoms and impact; the Insomnia Severity Index (ISI) was an exploratory endpoint.3
A total of 1,854 patients with chronic insomnia were randomised to QUVIVIQ™ or placebo treatment across two phase III trials, Study 1 and Study 2. 61% of participants were under 65 years old, two‑thirds were female and the mean time since insomnia diagnosis was over 10 years.3
The trials were composed of a 3‑month double‑blind treatment period, a 1‑week single‑blind placebo run‑out period, and then either a safety follow‑up (23 days) or a 9‑month placebo‑controlled extension.3
Placebo was deemed to be an appropriate comparator as chronic insomnia is classed as a non‑life‑threatening condition and no other treatments are licensed for long‑term use.6
The safety and efficacy of QUVIVIQ™ were evaluated in two multicentre,
randomised, double-blind, parallel-group, placebo-controlled Phase III studies3
- Click on the + icon to learn more
- Select inclusion criteria:
Age ≥18 years
Chronic insomnia (insomnia disorder) of moderate or severe intensity according to DSM-5 criteria (Insomnia Severity Index score ≥15 (clinical insomnia))
- Select exclusion criteria:
Any other condition causing insomnia
Ongoing cognitive behavioural therapy, shift work, travel over time zones
Treatment with central nervous system-active drugs for a minimum of 14 days prior to visit6*
Duration of treatment. The QUVIVIQ™ treatment duration should be as short as possible. The appropriateness of continued treatment should be assessed within 3 months and periodically thereafter.7
For full dosing considerations please refer to the Summary of Product Characteristics.
*Including over-the-counter medication and herbal medicines.6
†A 10 mg dose is not licensed for use and therefore no data are presented.7
DSM-5: Diagnostic and Statistical Manual of Mental Disorders, fifth edition
Quiz Summary
0 of 1 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Time has elapsed
Categories
- Not categorized 0%
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 1
1. Question
CorrectIncorrect
Ready for the next step?
Continue to the next topic to find out more about latency to persistent sleep
CBTi: cognitive behavioural therapy for insomnia; CI: confidence interval; CYP3A4: cytochrome P450 3A4; ISI: Insomnia Severity Index; LSM: least squares mean; LPS: latency to persistent sleep; NS: not statistically significant; SPC: Summary of Product Characteristics; STST: self‑reported total sleep time: WASO: wake time after sleep onset
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.7
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
- Ellis J, Ferini-Strambi L et al. Chronic insomnia disorder across europe: Expert opinion on challenges and opportunities to improve care. Healthcare (Basel) 2023;11(5)
- Hafner M, Romanelli R J et al. The societal and economic burden of insomnia in adults: an international study. RAND Corporation, 2023
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‑blind, placebo‑controlled, phase 3 trials. Lancet Neurol 2022;21(2):125‑139
- Campbell R, Chabot I et al. Understanding the unmet needs in insomnia treatment: a systematic literature review of real‑world evidence. Int J Neurosci 2023;133(8):864‑878
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 922. Daridorexant for treating long‑term insomnia, 18 October 2023. Available at: nice.org.uk. Accessed August 2025
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‑blind, placebo‑controlled, phase 3 trials. Lancet Neurol 2022;21(2):125‑139 (suppl)
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- Sleep Foundation. Sleep latency. Available at: sleepfoundation.org. Accessed August 2025
- Morin C M, Drake C L et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026
- Thomas D, Anderson W M. Multiple Sleep Latency Test (MSLT). Encyclopedia of sleep. Elsevier, 2013:96-99
- Sleep Foundation. Wakefulness after sleep onset. Available at: sleepfoundation.org. Accessed August 2025
- Zhu G, Catt M et al. Objective sleep assessment in >80,000 UK mid‑life adults: associations with sociodemographic characteristics, physical activity and caffeine. PLoS One 2019;14(12):e0226220
UK-DA-00655 | Date of preparation: September 2025

