QUVIVIQ™ practicalities
Home - Discover QUVIVIQ™ - QUVIVIQ™ Practicalities
Prescribing QUVIVIQ™: what you need to know
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.1
One tablet, once a night, every night1*
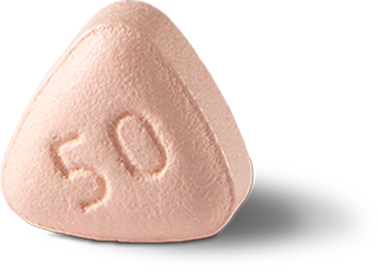
25 mg is recommended for patients with moderate hepatic impairment or taking moderate CYP3A4 inhibitors (eg erythromycin, ciprofloxacin, cyclosporine).1
In the case of co-administration with CNS-depressant medicinal products, dose adjustments of QUVIVIQ™ and/or the other medicinal products may be required, based on clinical evaluation, due to potentially additive effects.1
Upon discontinuation of QUVIVIQ™ in clinical trials, with up to 12 months of continuous nightly use, there was:1,4
no evidence of physical
dependence
no evidence of abuse or
withdrawal symptoms
no sign of rebound
insomnia
Special populations1
Elderly patients1
No dose adjustment is required in elderly patients (>65 years).
Because of the general risk of falls in the elderly, QUVIVIQ™ should be used with caution in this population, although clinical studies did not show an increase in the incidence of falls on QUVIVIQ™ compared with placebo.
QUVIVIQ™ should be administered with caution in patients older than 75 years since efficacy and safety data in this population are limited.
Patients with psychiatric comorbidities1
QUVIVIQ™ should be administered with caution in patients with psychiatric comorbidities since efficacy and safety data in this patient population are limited.
Patients with compromised respiratory function1
Patients with hepatic impairment1
QUVIVIQ™ is not recommended for use in patients with severe hepatic impairment.
In patients with moderate hepatic impairment the recommended dose is one 25 mg tablet once per night.
Contraindications1
- Hypersensitivity to the active substance or to any of the excipients
- Narcolepsy
- Concomitant use with strong CYP3A4 inhibitors (eg itraconazole, clarithromycin, ritonavir)
Other medications and side effects1
CNS-depressant effects
Because QUVIVIQ™ acts by reducing wakefulness, patients should be cautioned about engaging in potentially hazardous activities, driving or operating heavy machinery unless they feel fully alert, especially in the first few days of treatment. Caution should be exercised when prescribing QUVIVIQ™ concomitantly with CNS-depressant medicinal products due to potentially additive effects, and a dose adjustment of either QUVIVIQ™ or the concomitant CNS-depressants should be considered.
Sleep paralysis, hallucinations and cataplexy-like symptoms
Worsening of depression and suicidal ideation
Potential for abuse and dependence
There was no evidence of abuse or withdrawal symptoms indicative of physical dependence upon treatment discontinuation in clinical studies with QUVIVIQ™ in subjects with insomnia. In an abuse liability study of QUVIVIQ™ (50, 100 and 150 mg) conducted in non-insomniac recreational drug users (n=72), QUVIVIQ™ (100 and 150 mg) produced similar ‘drug liking’ ratings as zolpidem (30 mg). Because individuals with a history of abuse or addiction to alcohol or other substances may be at increased risk for abuse of QUVIVIQ™, these patients should be monitored carefully.
This medicine is subject to additional monitoring.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Idorsia at ds.safety.uk@idorsia.com.
This information is intended for UK healthcare professionals.
References
- QUVIVIQ™ (daridorexant) Summary of Product Characteristics
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1:935228
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- Sleep Foundation. Mastering sleep hygiene: your path to quality sleep. Available at: sleepfoundation.org. Accessed July 2025
UK-DA-00920 | Last updated: July 2025
QUV-Pro.idorsia QUVIVIQ Prescribing_June 2025

