QUVIVIQ™ resources
Home - Discover QUVIVIQ™ - QUVIVIQ™ Resources
QUVIVIQ™ is NICE recommended and SMC accepted for restricted use for the treatment of chronic insomnia in adults5,6
NICE recommendations5
QUVIVIQ™ is recommended for treating insomnia in adults with symptoms lasting for 3 nights or more per week for at least 3 months, and whose daytime functioning is considerably affected, only if:
- cognitive behavioural therapy for insomnia (CBTi) has been tried but not worked, or
- CBTi is not available or is unsuitable
SMC advice6
QUVIVIQ™ is accepted for restricted use within NHSScotland for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.
Restriction: in patients who have failed CBTi or for whom CBTi is unsuitable or unavailable.
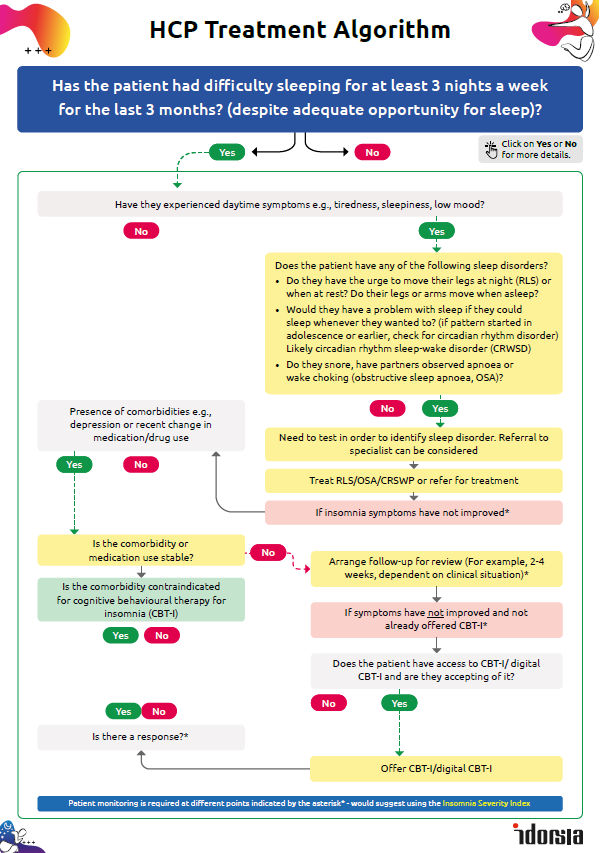
Is it time to prescribe QUVIVIQ™?
Has your patient had difficulty sleeping for at least 3 nights a week for the last 3 months (despite adequate opportunity for sleep)?
Follow the flowchart in this interactive treatment algorithm to find out what to do next.
*For best results, open in your desktop PDF viewer to enable functionality.
Assessing the severity of your patient's insomnia
The seven-question Insomnia Severity Index can be used to assess insomnia severity on a scale of ‘no clinically significant insomnia’ to ‘clinical insomnia (severe)’.7
How to support your patients after prescribing QUVIVIQ™
What do your patients know about chronic insomnia? Download this leaflet to give to your patients, providing information on:
- what insomnia means
- how to take QUVIVIQ™
- good sleep hygiene
- how QUVIVIQ™ works
†This resource is for patients prescribed QUVIVIQ™ only.
NICE: National Institute for Health and Care Excellence; SMC: Scottish Medicines Consortium
This medicine is subject to additional monitoring.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Idorsia at ds.safety.uk@idorsia.com.
This information is intended for UK healthcare professionals.
References
- QUVIVIQ™ (daridorexant) Summary of Product Characteristics
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1935228
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 922. Daridorexant for treating long-term insomnia, 18 October 2023. Available at: nice.org.uk. Accessed July 2025
- Scottish Medicines Consortium (SMC). SMC2611. Daridorexant film-coated tablets (Quviviq®), 8 April 2024. Available at: scottishmedicines.org.uk. Accessed July 2025
- Australasian Sleep Association. Insomnia severity index. Available at: sleepprimarycareresources.org.au. Accessed July 2025
© NICE 2023. Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/TA922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00921 | Last updated: July 2025
QUV-Pro.idorsia QUVIVIQ Resources_June 2025