Delayed management of chronic insomnia patients
"The most commonly perceived barrier to treatment is the perception of insomnia as benign, trivial, or a problem one should be able to cope with alone.1"
The current UK patients’ journeys are long and meandering, frequently repeating stages until they eventually resign themselves to a life with chronic insomnia.2
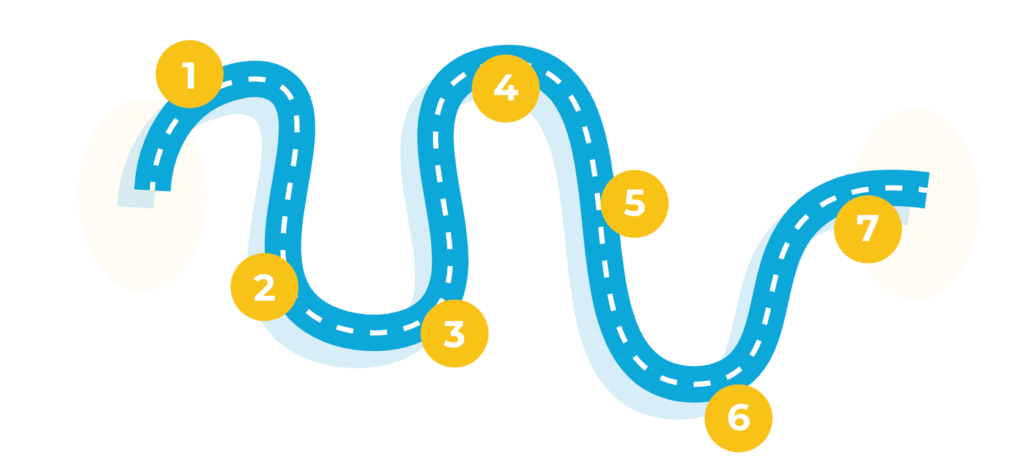
Onset of sleep issues
Self‑initiated behavioural change
OTC attempts to self‑manage
Onset of sleep issues
- 35%‑39% of the adult population suffer from insomnia symptoms; 7%‑15% are predicted to have chronic insomnia3
- Insomnia is often triggered by an acute life stressor and continues after the stressor has passed2
Self‑initiated behavioural change
- Patients begin researching about insomnia, frequently online4
- Information obtained from the internet often influences treatment decisions, such as how to manage insomnia and whether to seek professional care4
- The majority of the advice accessed online is not evidence-based and can perpetuate insomnia rather than resolve it4
- Behavioural changes may include sleep hygiene, screentime reduction, relaxation and mindfulness, diet and exercise changes, use of substances (alcohol and/or drug use/ abuse)2
OTC attempts to self‑manage
- Patients use OTC treatments, delaying the necessity to seek medical advice2
- Repeated use of ineffective OTC medication perpetuates confirmatory bias around maladaptive sleep‑promoting behaviours and beliefs5
- OTC misuse is of growing concern; misuse of sedating antihistamines contributes to drug‑related deaths6,7
First GP consultation
- When patients cannot keep up with daily responsibilities, a “crisis point” pushes them to seek help2
- Patients present at primary care when daytime functioning or mental and physical health become significantly impacted8
- A key barrier for patients is the expectation of their insomnia‑related symptoms not being taken seriously or no help being available; patients need to be really motivated to take this step1,8
Non‑pharmaceutical intervention, such as sleep hygiene and cognitive behavioural therapy for insomnia (CBTi)
- Depending on subjective assessment of the patient, most GPs will explore lifestyle changes before pharmaceutical interventions. In the UK, patients often receive sleep hygiene support, although GPs feel that patients 'switch off' to this advice9
- When sleep hygiene recommendations have not worked, GPs can refer patients for CBTi as a first‑line treatment for chronic insomnia in adults of any age,10,11 however, only 20%‑40% of GPs in England and Wales are able to refer patients for treatment due to a wide variation in local availability and funding,12 and only around half of these patients will adhere to the CBTi programme13
Current prescription initiation
- Until 2023 there was no standardised medication pathway; pharmacological choice is dependent on GP experience. GPs may prescribe short courses of hypnotics reluctantly; fear of dependence is common9
- Z-drugs and benzodiazepines are only recommended for patients with severe or acute exacerbation of insomnia and only for short duration (preferably less than 1 week). Tolerance reduces their effectiveness and may precipitate withdrawal symptoms10,14,15
Living with persistent insomnia
- In practice, many patients continue with prescription treatment longer than indicated2
- There has been an unmet need for a long-term pharmacological treatment option for chronic insomnia2
- QUVIVIQ™ is licensed for chronic insomnia in the UK and recommended by NICE* in adults with symptoms lasting for 3 nights or more per week for at least 3 months and whose daytime functioning is considerably affected.15
CBTi: cognitive behavioural therapy for insomnia; OTC: over‑the‑counter; Z-drug: zopiclone and zolpidem
Quiz Summary
0 of 1 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Time has elapsed
Categories
- Not categorized 0%
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 1
1. Question
CorrectIncorrect
Ready for the next step?
Patient preference for chronic insomnia treatment
CBTi: cognitive behavioural therapy for insomnia; OTC: over‑the‑counter
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.16
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- Stinson K, Tang N K, Harvey A G. Barriers to treatment seeking in primary insomnia in the United Kingdom: a cross-sectional perspective. Sleep 2006;29(12):1643-1646
- O’Regan D, Garcia-Borreguero D et al. Mapping the insomnia patient journey in Europe and Canada. Front Public Health 2023;11:1233201
- Hafner M, Romanelli R J et al. The societal and economic burden of insomnia in adults: an international study. RAND Corporation, 2023
- Moghe R, My Cheung J et al. Consumers using the internet for insomnia information: the who, what, and why. Sleep and Biological Rhythms 2014;12:297-304
- Cheung J M Y, Jarrin D C et al. Patterns of concomitant prescription, over-the-counter and natural sleep aid use over a 12-month period: a population based study. Sleep 2021;44(11):zsab141
- Gittins R, Missen L, Maidment I. Misuse of over the counter and prescription only medication by adults accessing specialist treatment services in the uk: a narrative synthesis. Subst Abuse 2022;16:11782218221111833
- Burns C. Rise in antihistamine-related deaths prompts call for move to POM status. The Pharmaceutical Journal 2021;306(7947); doi: 10.1211/PJ.2021.1.70125
- Ogeil R P, Chakraborty S P et al. Clinician and patient barriers to the recognition of insomnia in family practice: a narrative summary of reported literature analysed using the theoretical domains framework. BMC Fam Pract 2020;21(1):1
- Davy Z, Middlemass J, Siriwardena A N. Patients’ and clinicians’ experiences and perceptions of the primary care management of insomnia: qualitative study. Health Expect 2015;18(5):1371-1383
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing long-term insomnia (more than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 2022;21(2):125-139
- M3 Idorsia insomnia market research. May 2022 GP Omnibus results (N=1, 002 UK GPs; update 22 June 2022)
- Koffel E, Bramoweth A D, Ulmer C S. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med 2018;33(6):955-962
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Scenario: managing short-term insomnia (less than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 922. Daridorexant for treating long‑term insomnia, 18 October 2023. Available at: nice.org.uk. Accessed August 2025
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- Heidenreich S, Ross M et al. Preferences of patients for benefits and risks of insomnia medications using data elicited during two phase III clinical trials. Sleep 2022;45(11):zsac204
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00656 | Date of preparation: September 2025

