Dosing

Dosing
QUVIVIQ™ 50 mg may be suitable for your adult patients with chronic insomnia
Dosing
QUVIVIQ™ works differently to other hypnotics, reducing overactive wake signalling and allowing restorative sleep to occur without altering the proportion of sleep stages.1-7
The recommended dose for adults is:
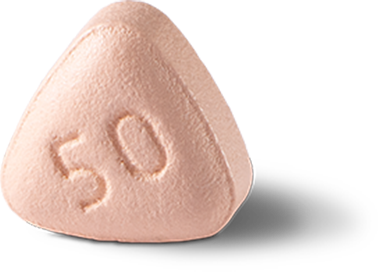
The recommended dose for patients with moderate hepatic impairment or taking moderate CYP3A4 inhibitors is:

Elderly
No dose adjustment is required in elderly patients (>65 years). Because of the general risk of falls in the elderly, QUVIVIQ™ should be used with caution in this population, although clinical studies did not show an increase in the incidence of falls on QUVIVIQ™ compared with placebo.1
QUVIVIQ™ should be administered with caution in patients >75 years since efficacy and safety data in this population are limited.1
No data are available in patients >85 years.1
Do not prescribe to patients if they are taking medications which may increase the level of QUVIVIQ™ in the blood such as:1
- triazole antifungals
- macrolide antibiotics
- HIV protease inhibitors
- small module kinase inhibitors for cancer treatment
Interaction studies have only been performed in adults. There is no data available in paediatric populations.1
Contraindications
QUVIVIQ™ is contraindicated in patients with:1
- hypersensitivity to the active substance or to any of the excipients
- narcolepsy
- concomitant use of strong CYP3A4 inhibitors
Special considerations:
CNS-depressant effects
Because QUVIVIQ™ acts by reducing wakefulness, patients should be cautioned about engaging in potentially hazardous activities, driving or operating heavy machinery unless they feel fully alert, especially in the first few days of treatment.1
Caution should be exercised when prescribing QUVIVIQ™ concomitantly with CNS‑depressants due to potentially additive effects, and a dose adjustment of either QUVIVIQ™ or the concomitant CNS‑depressant should be considered.1
Available data from a lactation study in 10 healthy lactating women receiving 50 mg daridorexant indicates that the presence of daridorexant in breast milk is low, with a fraction of the maternal dose of daridorexant excreted into breast milk of 0.02%.1
A risk of excessive somnolence to the breastfed infant cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/ abstain from QUVIVIQ™ therapy, taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.1
Daridorexant did not increase the frequency of apnoea/ hypopnoea events or cause oxygen desaturation in patients with mild-to-moderate (5 to <30 events per hour of sleep) or severe (≥30 events per hour of sleep) obstructive sleep apnoea (OSA). Nor did it cause oxygen desaturation in patients with moderate COPD. Daridorexant has not been studied in patients with severe COPD (FEV1 <40% of predicted).1
Caution should be exercised when prescribing QUVIVIQ™ to patients with severe COPD.1
QUVIVIQ™ should be administered with caution in patients with psychiatric comorbidities since efficacy and safety data in this patient population are limited.1
Use is not recommended in patients with severe hepatic impairment. The recommended dose of QUVIVIQ™ for patients with moderate hepatic impairment is 25 mg once per night.1
In patients with renal impairment (including severe), no dose adjustment is required.1
Individuals with a history of abuse or addiction to alcohol or other substances may be at increased risk for abuse of QUVIVIQ™; these patients should be followed carefully.1
There was no evidence of abuse or withdrawal symptoms indicative of physical dependence upon treatment discontinuation in clinical studies with QUVIVIQ™ in subjects with insomnia.1
In primarily depressed patients treated with hypnotics, worsening of depression and suicidal thoughts and actions have been reported. As with other hypnotics, QUVIVIQ™ should be administered with caution in patients exhibiting symptoms of depression. Isolated cases of suicidal ideation have been reported in phase III clinical studies, in subjects with pre‑existing psychiatric conditions and/or stressful living conditions, across all treatment groups, including placebo. Suicidal tendencies may be present in patients with depression and protective measures may be required.1
Symptoms similar to mild cataplexy have been reported with DORAs.1
Prescribers should explain the nature of these events to patients when prescribing QUVIVIQ™. Should such events occur, patients need to be further evaluated and, depending on the nature and severity of the events, discontinuation of treatment should be considered.1
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume (in 1 second)
Please consult the Summary of Product Characteristics for a complete list of prescribing conditions and adverse events.
What patients need to know about dosing:
QUVIVIQ™ is taken every night, regardless of how tired they feel1
QUVIVIQ™ should be taken within 30 minutes before going to bed1
If they forget to take QUVIVIQ™ before bed, that dose should not be taken during the night1
Time to sleep onset may be delayed if taken soon after a large meal1
Patients should continue to practise good sleep hygiene8
Patients should not drink alcohol with QUVIVIQ™ and should avoid grapefruit juice in the evenings1
For patients who have been prescribed QUVIVIQ™, you can download a patient information sheet HERE
Managing patient expectations
Quiz Summary
0 of 1 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Time has elapsed
Categories
- Not categorized 0%
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 1
1. Question
CorrectIncorrect
CYP3A4 : cytochrome P450 3A4; DORA: dual orexin receptor antagonist; OSA: obstructive sleep apnoea
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.1
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- Roch C, Bergamini G et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl) 2021;238:2693-2708
- Chaput J P, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep 2018;10:421-430
- Di Marco T, Djonlagic I et al. Effect of daridorexant on sleep architecture in patients with chronic insomnia disorder: a pooled post hoc analysis of two randomized Phase 3 clinical studies. Sleep 2024:doi.org/10.1093/sleep/zsae1098
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1:doi:10.3389/frsle.2022.935228
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‑blind, placebo‑controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing long-term insomnia (more than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- Stinson K, Tang N K, Harvey A G. Barriers to treatment seeking in primary insomnia in the United Kingdom: a cross-sectional perspective. Sleep 2006;29(12):1643-1646
- Morin C M, Drake C L et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing short-term insomnia (less than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. How should I assess a person with suspected insomnia? May 2025. Available at: cks.nice.org.uk. Accessed August 2025
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00656 | Date of preparation: September 2025
