Differentiating chronic insomnia from other sleep‑related disorders

Differentiating chronic insomnia from other sleep‑related disorders
Parasomnias
Characterised by unusual or unpleasant experience or behaviours associated with sleep which are troublesome or dangerous. Examples include sleepwalking, sleep paralysis and night terrors9
- occurs frequently in the general population, with >30% of individuals experiencing at least one type of parasomnia10
Obstructive sleep apnoea syndrome
A sleep-related breathing disorder characterised by complete or partial upper airway obstruction during sleep11
- occurs in ~13% of men and 6% of women aged 30-70 years12
Circadian rhythm disorders
Includes delayed and advanced sleep-wake disorder and irregular sleep-wake rhythm disorder, characterised by persistent or recurrent sleep disturbance due to changes in circadian system affecting timing or duration of sleep13
- between 3%-10% of the population may be affected14
Restless legs syndrome (Willis-Ekbom disease)
Characterised by an irresistible urge to move the limbs, usually the legs, alongside paraesthesia-like sensations (tingling, crawling, cramping or aching). These symptoms are usually worse in the evenings15
- occurs in 2%-15% of the population12
Narcolepsy
Characterised by sleep attacks (falling asleep in the daytime without warning) and cataplexy (temporary loss of muscle control causing weakness/ collapse, often triggered by emotion)16
- a rare disorder, occuring in <0.1% of the adult population17
Risk factors for insomnia: the 3P behavioural model
The 3P behavioural model of insomnia describes the course of insomnia, from acute through to chronic, and self‑perpetuating due to the interaction of three types of risk factors.7,18
Learn more
Three types of risk factors
1 – Predisposing factors, such as age, sex, anxiety‑prone personalities and genetic factors increase vulnerability to insomnia7
Prevalence is 1.5-2X greater in females vs males8,19,20
Can occur at any age but more common in older adults8,19
Prevalence is higher with comorbidities including COPD, heart failure, depression, anxiety and PTSD8
2 – Precipitating factors, ie acute occurrences that trigger a sleep disturbance, such as occupational stress, illness or a separation7,18
3 – Perpetuating factors, ie behaviours to compensate/ cope with sleeplessness, which stop the resumption of normal sleep patterns after an acute trigger, such as:18
non-sleep activities in the bedroom
staying in bed when awake
spending excessive time in bed (eg naps/ early bedtimes)

Tools for diagnosis
The first step in diagnosis is obtaining a detailed patient history. This may include:8
- symptoms (eg difficulty getting to sleep, maintaining sleep, early wakening, non-restorative sleep or daytime impairment)
- duration and frequency of symptoms
- sleep schedule (eg light out time, time taken to fall asleep, number of nightly awakenings, rise time)
- sleep environment
- possible triggers (eg stress)
- behaviours during sleep that may indicate an alternative cause, such as snoring, witness apnoea, restless legs or sleepwalking
- relevant medical history such as comorbidities and previous insomnia treatments
- medications/ substance use (including caffeine, alcohol, nicotine, sedatives)
- impact of insomnia on quality of life (including mood, employment, relationships etc)
Validated self‑reporting tools remain the most practical methods for the assessment of insomnia in clinical practice:
- Click on the tools below to open in a new window
Sleep diary21
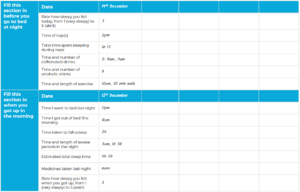
- A useful way of confirming patient sleep history and monitoring for change
Epworth sleepiness scale22
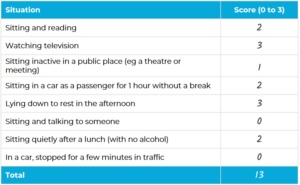
- Assesses how likely a patient is to fall asleep during various activities
- Can help determine when to refer for further investigation
Insomnia severity index (ISI)21,23

- A seven-question survey to gauge severity of insomnia and evaluate treatment response
These can be used both when sleep difficulty is the major presenting condition and when sleep difficulty is suspected secondary to the presenting condition.
Sleep studies
In order to exclude/ confirm other sleep‑related conditions including sleep apnoea and restless legs syndrome, referral for more detailed sleep studies may be required.7,19
Quiz Summary
0 of 1 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Time has elapsed
Categories
- Not categorized 0%
-
Sleep studies
In order to exclude/ confirm other sleep‑related conditions including sleep apnoea and restless legs syndrome, referral for more detailed sleep studies may be required.7,19
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 1
1. Question
CorrectIncorrect
Ready for the next step?
Treating chronic insomnia: the patient journey
COPD: chronic obstructive pulmonary disorder; DSM-5-TR: Diagnostic and Statistical Manual of Mental Disorders 5th edition text revision; ICD-11: International Classification of Diseases 11th Revision; ICSD-3 TR: International Classification of Sleep Disorders text revision, Diagnostic and Coding Manual 3rd edition; NICE: National Institute for Health and Care Excellence; PTSD: post-traumatic stress disorder; WHO: World Health Organization
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.24
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- World Health Organization (WHO). International statistical classification of diseases and related health problems (ICD) 11th revision. Available at: icd.who.int. Accessed August 2025
- Sharpley A L, Attenburrow M E, Cowen P J. Assessment and treatment of insomnia (including a case control study of patients with primary insomnia). Int J Psychiatry Clin Pract 1997;1(2):107-117
- Riemann D, Baglioni C et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26(6):675-700
- Hafner M, Romanelli R J et al. The societal and economic burden of insomnia in adults: an international study. RAND Corporation, 2023
- Judd B G, Sateia M J. Classification of sleep disorders, 2022. Available at: uptodate.com. Accessed August 2025
- Mai E, Buysse D J. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin 2008;3(2):167-174
- Morin C M, Benca R. Chronic insomnia. Lancet 2012;379(9821):1129-1141
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia, June 2024. Available at: cks.nice.org.uk. Accessed August 2025
- Sleep Foundation. Parasomnias. Available at sleepfoundation.org. Accessed August 2025
- Ohayon M M. The parasomnias and other sleep-related movement disorders (Editors Thorpy M J, Plazzi G). Chapter 2 – Epidemiology of parasomnias. Cambridge University Press 2010:7-12
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Obstructive sleep apnoea syndrome. Scenario: management of sleep apnoea, November 2021. Available at: cks.nice.org.uk. Accessed August 2025
- Parliamentary Office of Science and Technology (POST). 2018. POSTbrief 29: sleep and long-term health. Available from researchbriefings.files.parliament.uk. Accessed August 2025
- Sleep Foundation. Circadian rhythm disorders. Available at sleepfoundation.org. Accessed August 2025
- Lee E K. Circadian rhythm sleep-wake disorders (Editor Auger R R). Introduction to circadian rhythm disorders. Springer, 2020:29-43
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Restless legs syndrome, August 2024. Available at: cks.nice.org.uk. Accessed August 2025
- NHS. Narcolepsy, December 2022. Available at: nhs.uk. Accessed August 2025
- Narcolepsy UK. About narcolepsy. Available from narcolepsy.org.uk. Accessed August 2025
- Perlis M, Shaw P et al. Models of Insomnia. In M H Kryger, T Roth, W C Dement, eds, Principles and practice of sleep medicine, 5th edition, 2010, 850-865
- Morin C M, Drake C L et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026
- Wilson S, Anderson K et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol 2019;33(8):923-947
- Riemann D, Espie C A et al. The European Insomnia Guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res 2023;32(6):e14035
- The Epworth Sleepiness Scale. Available at: nasemso.org. Accessed August 2025
- Morin C M, Belleville G et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601-608
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00653 | Date of preparation: September 2025
