Prescribing QUVIVIQ™: what you need to know
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.5
The recommended dose for adults is 50 mg once per night5

25 mg once‑nightly dosing is recommended for patients with moderate hepatic impairment or taking moderate CYP3A4 inhibitors (eg erythromycin, ciprofloxacin or ciclosporin)5
QUVIVIQ™ is NICE-recommended for treating insomnia in adults with symptoms lasting for 3 nights or more per week for at least 3 months, and whose daytime functioning is considerably affected, only if:6
- cognitive behavioural therapy for insomnia (CBTi) has been tried but not worked, or
- CBTi is not available or is unsuitable
The length of treatment should be as short as possible. Treatment with daridorexant (QUVIVIQ™) should be assessed within 3 months of starting and should be stopped in people whose long-term insomnia has not responded adequately. If treatment is continued, assess whether it is still working at regular intervals.6
Proposed treatment algorithm for chronic insomnia following availability of QUVIVIQ™
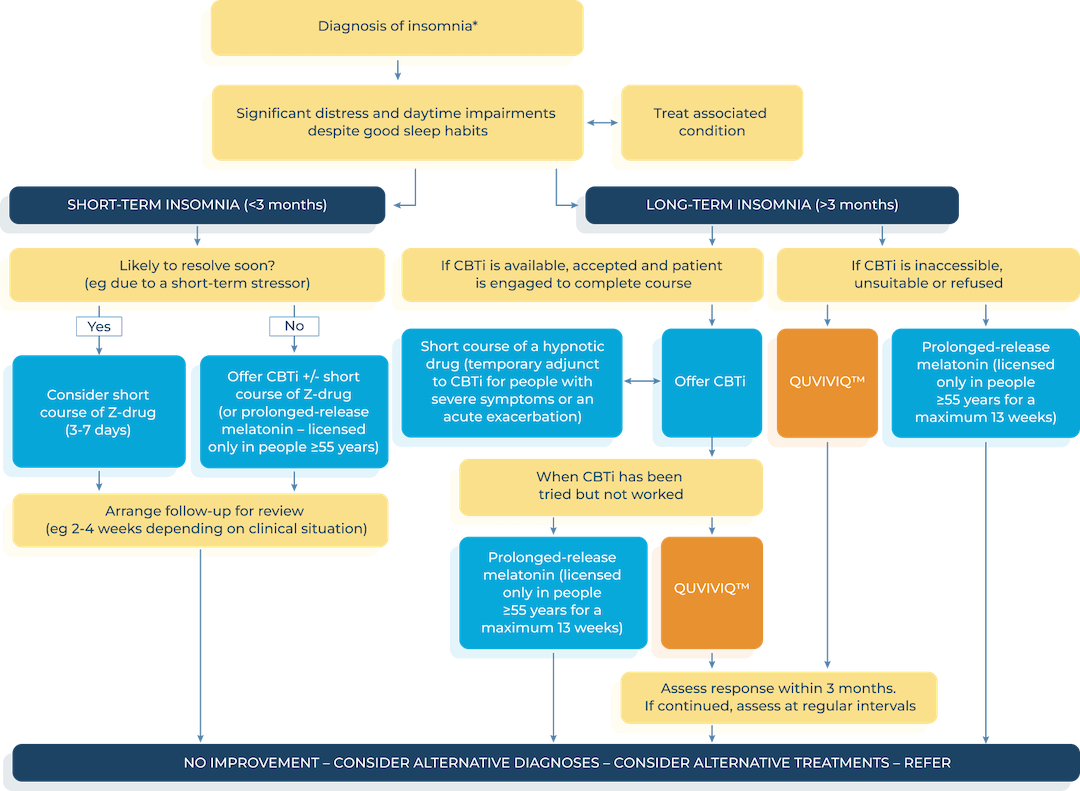
*Consider the need for referral to a sleep clinic or neurology if symptoms of another sleep disorder are present. Address circumstances/ stressors associated with onset of insomnia. Ensure comorbidities (eg anxiety or depression) are optimally managed. Offer advice on sleep hygiene. Advise the person not to drive if they feel sleepy.
Switching insomnia treatments
- BZ dose should be tapered and CBTi provided17
- Z-drugs should be tapered with a 1‑2 day delay before administration of the next insomnia therapy17
- Off-label antidepressants should also be gradually tapered17
- There is no need to taper the dose of doxepin or DORAs17
Ready for the next step?
BAP: British Association for Psychopharmacology; CBTi: cognitive behavioural therapy for insomnia; CYP3A4: cytochrome P450 3A4; DORA: dual orexin receptor antagonist; EIN: European Insomnia Network; ESRS: European Sleep Research Society; GABA: gamma-aminobutyric acid; NICE: National Institute for Health and Care Excellence; OTC: over-the-counter; Z-drug: nonbenzodiazepines
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.5
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- Wilson S, Anderson K et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol 2019;33(8):923-947
- O’Regan D, Garcia-Borreguero D et al. Mapping the insomnia patient journey in Europe and Canada. Front Public Health 2023;11:1233201
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing short-term insomnia (less than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia. Scenario: managing long-term insomnia (more than 3 months duration), May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 922. Daridorexant for treating long-term insomnia, 18 October 2023. Available at: nice.org.uk. Accessed August 2025
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Insomnia, May 2025. Available at: cks.nice.org.uk. Accessed August 2025
- Riemann D, Espie C A et al. The European Insomnia Guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res 2023;32(6):e14035
- Prolonged-release melatonin Summary of Product Characteristics
- British National Formulary (BNF). Melatonin. Available from bnf.nice.org.uk, Accessed August 2025
- Roch C, Bergamini G et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl) 2021;238(10):2693-2708
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1:935228
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- Chaput J P, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep 2018;10:421-430
- Di Marco T, Djonlagic I et al. Effect of daridorexant on sleep architecture in patients with chronic insomnia disorder – a pooled post hoc analysis of two randomized phase 3 clinical studies. Sleep 2024:zsae098
- Watson N F, Benca R M et al. Alliance for sleep clinical practice guideline on switching or deprescribing hypnotic medications for insomnia. J Clin Med 2023;12(7):2493
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00653 | Date of preparation: September 2025

