QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.1
Understanding wakefulness and the orexin system
Home - Discover QUVIVIQ™ - Wakefulness
The overactive brain - pathophysiology of insomnia
The timing of sleep is regulated by two processes:5
- Homeostatic process - sleep debt (or ‘pressure’) which increases during wakefulness and decreases during sleep
- Circadian process - the drive for wakefulness, which increases through the day, peaks during early evening and suddenly subsides at the beginning of the night. Active promotion of sleep occurs later in the night
The ascending reticular activating system (a series of brainstem nuclei; ARAS) regulates wakefulness by releasing neurotransmitters, eg acetylcholine, noradrenaline, dopamine, serotonin and histamine.6,7
The neuropeptide orexin further maintains wakefulness by reinforcing the activity of the wake‑promoting neurotransmitters involved in ARAS during the day.7-10
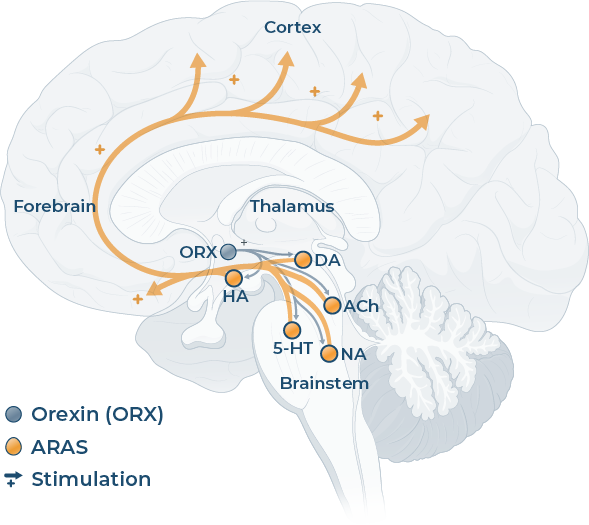
Adapted from Saper C B et al, 20057*
Sleep pressure (debt) accumulates during the day and secretion of melatonin and neurotransmitters from the inhibitory sleep system downregulate key areas of ARAS.7,11
Activity of orexin-releasing neurons also decreases, resulting in reduced stimulation of the ARAS and promoting sleep.7,8,11
Orexin neurons are typically inactive at night9
Adapted from Saper C B et al, 20057*
Multiple studies suggest that wake‑promoting regions of the brain remain overactive at night.12,13
Overactive wake‑signalling is also known as hyperarousal.12-14
Since orexin promotes wakefulness, it could be a contributory factor to the state of chronic insomnia.8
In chronic insomnia, orexin neurons may stay active at night9,10,13
Adapted from Saper C B et al, 20057*
*Images are representative of states in the brain and the principal areas involved – they are not intended to be comprehensive of all brain areas involved.
5-HT: serotonin; ACh: acetylcholine; DA: dopamine; HA: histamine; NA: noradrenaline; ORX: orexin
Adapted from Saper C B et al, et al. 20057
Since orexin is involved in wake-signalling, orexin receptors provide a specific target for therapeutic intervention10
QUVIVIQ™ reduces overactive wake-signalling by blocking orexin, allowing restorative sleep to occur without altering the proportion of sleep stages.1-4,10,15,16
This medicine is subject to additional monitoring.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Idorsia at ds.safety.uk@idorsia.com.
This information is intended for UK healthcare professionals.
References
- QUVIVIQ™ (daridorexant) Summary of Product Characteristics
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 2022;21:125-139
- Robbins R, Quan S F et al. A nationally representative survey assessing restorative sleep in US adults. Front Sleep 2022;1:935228
- Kunz D, Dauvilliers Y et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 2023;37:93-106
- della Monica C, Dijk D J. What makes a good night’s sleep? The external and internal factors that influence a good night’s sleep. Physiol News 2018:36-39
- Phillips A J, Robinson P A. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. J Biol Rhythms 2007;22:167-179
- Saper C B, Scammell T E, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437:1257-1263
- Brisbare-Roch C, Dingemanse J et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 2007;13:150-155
- Scammell T E, Winrow C J. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol 2011;51:243-266
- Roch C, Bergamini G et al. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl) 2021;238:2693-2708
- Saper C B, Chou T C, Scammell T E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726-731
- Nofzinger E A, Buysse D J et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004;161:2126-2128
- Riemann D, Spiegelhalder K et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 2010;14:19-31
- Morin C M, Drake C L et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026
- Di Marco T, Djonlagic I et al. Effect of daridorexant on sleep architecture in patients with chronic insomnia disorder: a pooled post hoc analysis of two randomized Phase 3 clinical studies. Sleep 2024:zsae098
- Chaput J P, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep 2018;10:421-430
UK-DA-00916 | Last updated: July 2025
QUV-Pro.idorsia QUVIVIQ Wakefulness_June 2025

