Understanding chronic insomnia – a self-led learning programme
A comprehensive, expert-approved e-learning programme for healthcare professionals.
- Educational support in accordance with NICE TA922
- Proof of learning
You already have an account?
These modules have been reviewed by an expert faculty in sleep and insomnia. Content may be reflective of their clinical experience and opinion.
Dr Kirstie Anderson, Consultant Neurologist
Paediatric and adult neurological sleep service lead at Newcastle Hospitals NHS Foundation Trust
Dr Hugh Selsick, Consultant Psychiatrist
Founded and led the Insomnia and Behavioural Sleep Medicine Clinic at UCLH. Director of Sleep 360
Also published on

What you will learn
The ‘Understanding chronic insomnia‘ self‑led learning programme comprises six 20‑minute modules, allowing you to enhance your knowledge of:
- identifying and managing patients with chronic insomnia
- the impact of chronic insomnia beyond night sleeping
- selecting appropriate treatments per individual to address insomnia‑related symptoms
Chronic insomnia is a prevalent condition characterised by persistent difficulty with the onset and maintenance of sleep or unintentional early-morning awakening, manifesting at least 3 nights per week for at least 3 months.1 Many people struggle with the symptoms and health consequences of untreated insomnia.2 QUVIVIQ™▼(daridorexant), recommended second‑line after CBT for insomnia, represents an alternative for eligible patients to more traditional treatments for insomnia such as benzodiazepine receptor agonists, antihistamines and melatonin receptor agonists.3-5
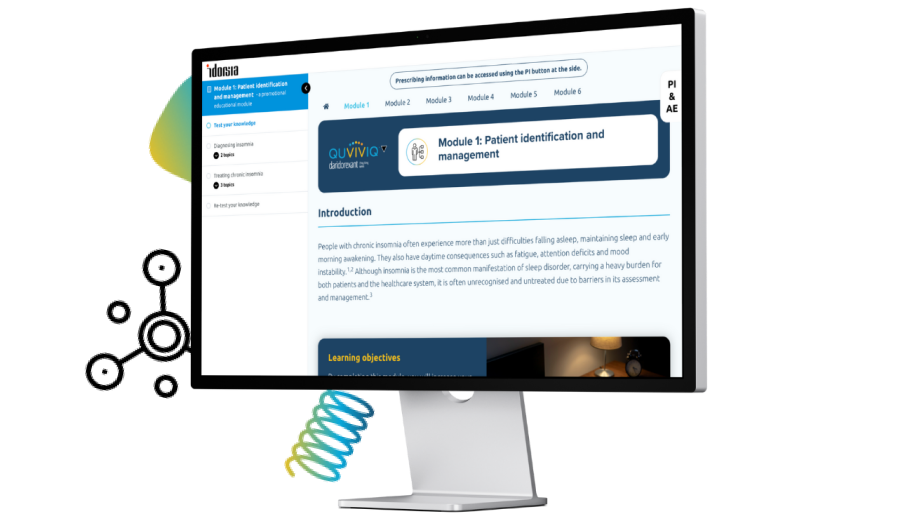
Course content
You will be able to assess your knowledge in each subject and download a learning certificate for your records.
Select a module to begin.
Module 1: Patient identification and management
- How to recognise chronic insomnia, including differential diagnoses and diagnostic tools
- Overview of the clinical treatment landscape
- Identifying patients who could benefit from QUVIVIQ™
Module 2: Sleep mechanisms and QUVIVIQ™ action
- The pathophysiology of insomnia, including the interplay of wake- and sleep‑promoting systems
- The mechanism of action of QUVIVIQ™ compared with more traditional treatments for insomnia, and how that affects its use
Module 3: Clinical data for QUVIVIQ™
- The unmet need for appropriate treatments for patients with chronic insomnia6,7
- Clinical data for night-time and daytime insomnia symptoms for QUVIVIQ™ versus placebo
Module 4: Addressing patient concerns
- The patient journey for those suffering from insomnia
- How patient preferences come into play in insomnia management
- Counselling patients about QUVIVIQ™ use
Module 5: QUVIVIQ™ in practice: patient cases
- Example patient case studies to exemplify where QUVIVIQ™ could offer a benefit:
- insomnia in a postmenopausal female
- managing insomnia in older patients
Module 6: The impact of insomnia
- The direct and indirect impact of chronic insomnia on:
- individual patients
- the wider community
- public health
- the economic climate
Get Started
Enhance your knowledge on
chronic insomnia today
You already have an account?
CBT: cognitive behavioural therapy; NICE TA922: National Institute for Health and Care Excellence Technology Appraisal Guidance TA922
QUVIVIQ™ is indicated for the treatment of adult patients with insomnia characterised by symptoms present for at least 3 months and considerable impact on daytime functioning.5
This information is intended for UK healthcare professionals.
Adverse events must be reported. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to ds.safety.uk@idorsia.com
References
- Nyhuis C C, Fernandez‑Mendoza J. Insomnia nosology: a systematic review and critical appraisal of historical diagnostic categories and current phenotypes. J Sleep Res 2023;32(6):e13910
- Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med 2007;3(5 Suppl):S7‑10
- Robinson C L, Supra R et al. Daridorexant for the treatment of insomnia. Health Psychol Res 2022;10(3):37400
- NICE. TA922. Daridorexant for treating long-term insomnia, October 2023. Available at: https://www.nice.org.uk/guidance/ta922. Last accessed September 2025.
- QUVIVIQ™ Idorsia Pharmaceuticals Ltd, Summary of Product Characteristics
- Campbell R, Chabot I et al. Understanding the unmet needs in insomnia treatment: a systematic literature review of real‑world evidence. Int J Neurosci 2023;133(8):864‑878
- Mignot E, Mayleben D et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‑blind, placebo‑controlled, phase 3 trials. Lancet Neurol 2022;21(2):125‑139
© NICE 2023 Daridorexant for treating long-term insomnia. Available from www.nice.org.uk/guidance/ta922. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/ publication.
UK-DA-00652 | Date of preparation: September 2025
